DIAGNOSTICS
BACKGROUND
There is no question that a diagnosis of any cancer is often devastating to patients and their families. Most patients are asymptomatic until they present with an advanced disease when the prognosis is worst. According to the American Cancer Society, patients diagnosed with early stage bladder cancer have 5-year survival rates of >90%, compared to approximately 15-30% for patients diagnosed in advanced stage, and drops off sharply to <5% for metastatic disease. The development of a biomarker that detects BC earlier would be a very important advance. Equally important, such a marker could be used to follow patients’ post-surgical resection and monitor their response to therapy. A test that would provide for early detection of recurrence or progression would be useful because it could lead to the initiation of systemic treatment sooner than current methods allow. A marker that plays an important role in tumorigenesis would be preferred because loss of the marker after treatment may indicate less aggressive disease.
We identified in serum/plasma of bladder and kidney cancer patients a protein called QSOX1-L from >200 patient samples, which was low or non-existent in normal donor samples. Two forms of QSOX1 exist, the long and short forms. We found that tumors preferentially secrete the long form of QSOX1 (QSOX1-L) into circulation. QSOX1-L is known to be present differentially in most tumor tissues and, as we recently learned, in blood of BC patients.
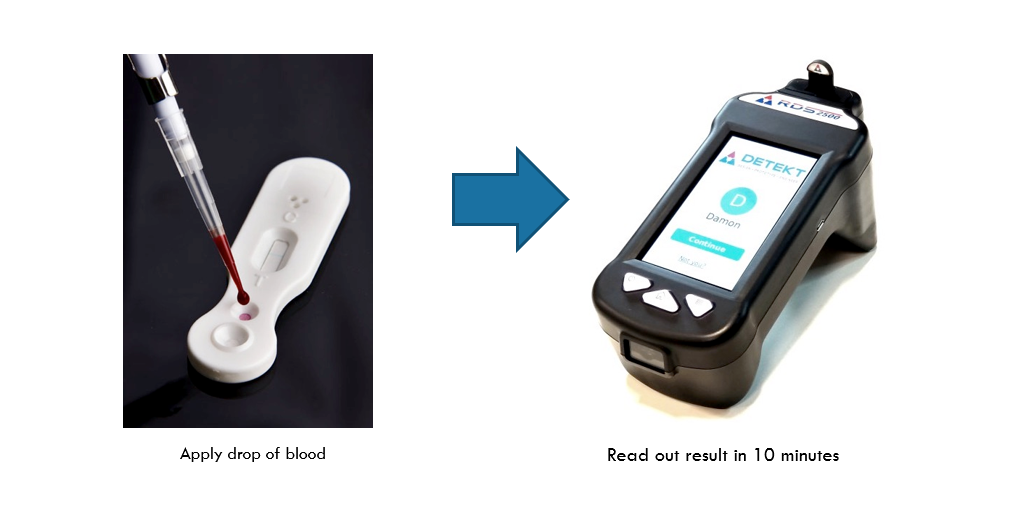
Our overall objective is to develop a low cost non-invasive Rapid Diagnostic Test (RDT) for the detection of BC from urine based on an enzyme biomarker QSOX1 overexpressed in most cancers 1, including bladder, with which we will test urine samples from control and test populations. Our hypothesis is that (i) QSOX1-L or QSOX1-related polypeptides can also be shed from BC urothelium into urine; (ii) patients with BC will show elevated levels of QSOX1-L in urine compared to patients with non-malignant diseases and (iii) QSOX1-L levels will decrease post-surgery and/or with medical treatment and rise with recurrence of tumor or progression of disease.
Background: Cancer remains one of the leading causes of morbidity and mortality worldwide. According to the WHO, cancer burden had risen to 18.1 million new cases and 9.6 million cancer deaths in 2018.2 While it’s well known that cancer is a leading cause of death and disability worldwide, what is less recognized is the significant growth of cancer in the developing world.3 Only two decades ago, the percentage of new cases was similar for developed and developing regions. Today, 55 percent of new cases arise in developing nations—a figure that could reach 60 percent by 2020 and 70 percent by 2050.4 These disparities in cancer risk combined with poor access to epidemiological data, research, treatment, and cancer control and prevention combine to result in significantly poorer survival rates in developing countries for a range of malignancies.
BC ranks 13th in terms of number of deaths, with mortality rates decreasing particularly in the most developed countries.5 The exceptions are countries undergoing rapid economic transition, including in Central and South America, China, central, southern, and eastern European countries.5 The observed patterns and trends of BC incidence worldwide appear to reflect the prevalence of tobacco smoking.3 This contrasts with steady but slow decrease in smoking rates in industrialized nations. Emerging evidence also suggests that environmental factors such as chlorinated water may account for large number of new BC cases.6 Infection with Schistosoma haematobium is a well-documented risk factor and an important cause of BC in developing world.7 Early detection and access to advanced diagnostic modalities and cancer therapies has led to declines in the incidence and mortality of BC in developed countries not seen in less developed communities.
Significance: The presenting feature of many new BC cases is haematuria, and diagnostic work up for haematuria includes cystoscopy and upper tract imaging to detect urinary tract malignancies. Diagnosis of BC has not evolved considerably over the past decades. Cytoscopy remains the gold standard for the detection and follow up of BC.8 Cystoscopy is highly sensitive in the detection of most bladder tumors with reported sensitivities of approximately 90%.9 It is however an expensive and invasive procedure that incites anxiety and causes discomfort in patients undergoing the test that often results in adverse effects such as infection, frequency, dysuria, and visible hematuria.10,11 Last, high recurrence and the frequent need for follow-up impose a very high financial burden on patients and their families. Non-invasive urine cytology, although effective in detecting high-grade tumors (75% sensitivity), is severely limited in the diagnosis of low-grade malignancies (25% sensitivity).12 Therefore, developing cost-effective and non-invasive strategies for the detection of BC is of paramount importance particularly in the low resource settings because of the high cost of cystoscopy.
Urinary biomarkers can be useful diagnostic tool in BC as urine-based diagnostics offer a non-invasive and cost-effective means for BC detection. Despite significant progress in discovering differentially expressed urinary protein markers,9 there are only a few FDA approved commercial rapid tests on the market today. All these tests however lack sensitivity and specificity required to qualify as a screening tool for BC detection. For example, Bladder Tumor Antigen (BTA Stat® test by Bion Diagnostic Sciences, Redmond, WA) has low specificity due to benign genitourinary conditions; also it gives a lot of false positives in patients with hematuria.9 BladderChek® test marketed by Alere detects a specific nuclear matrix protein NMP22 with a sensitivity of 49%–65% % and a specificity of 40%–90% %.13,14 The high variability of NMP22 means that it is not ideal for rapid, easy detection of BC. Similar to BTA, NMP22 sensitivity is impacted by other non-cancerous conditions such as hematuria or inflammation.15 The FDA approved this test as an aid in the diagnosis of patients at risk or with symptoms of BC. Another test, UBC® Rapid Test, measures soluble fragments of cytokeratins 8 and 18 in urine.16 Assays based on cytokeratins detection are limited by relatively high false positive rates and limited ability to detect low grade tumors.17 Although all tests mentioned above outperform cytology, none of them have been widely adopted by urologists14 and thus, their application has not reduced the need for cystoscopy. Application of multi-marker panels is widely seen as viable solution to the problematic statistical performance of any single biomarker.8
Clearly, finding new BC biomarkers that alone (or in a combination with other biomarkers) yield non-invasive test that performs as well or better than cystoscopy would be a very significant new development.