DIAGNOSTICS
OVERVIEW
Our diagnostic research is driven by our discovery of the QSOX1-L biomarker that is highly specific for cancer and is low or non-existent in healthy individuals. The quantitative assays for QSOX1-L will allow us to determine if its levels correlate with disease stage, recurrence or response to therapy. Our diagnostic research combines three innovations: (a) Novel cancer biomarker; (b) Generation of novel antibodies that selectively detect this biomarker; and (c) Use of this biomarker to develop a diagnostic test for cancer.
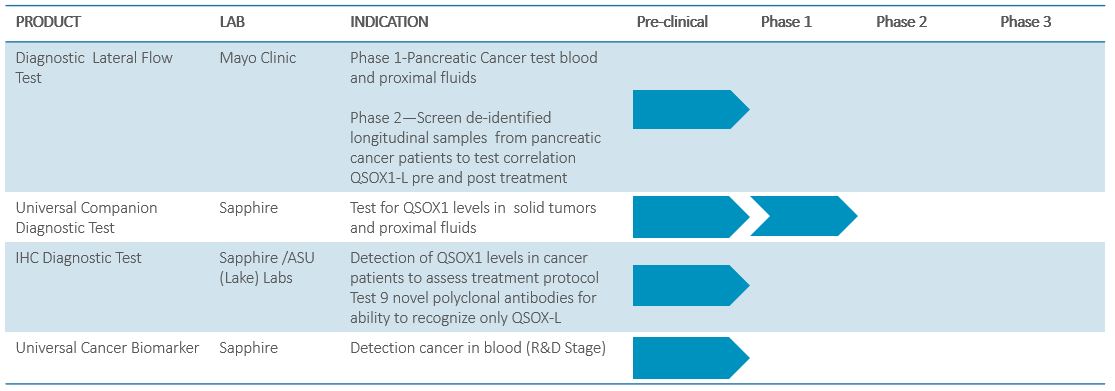
DIAGNOSTICS PIPELINE
BACKGROUND
SIGNIFICANCE AND INNOVATION
Bladder cancer (BC) is one of the most common cancers worldwide seeing steady increase in developing countries. BC detection traditionally requires cystoscopy, an unpleasant and expensive surgical procedure, which is often accompanied by adverse side effects. An unmet clinical need exists for accurate, noninvasive assays, which enable the accurate diagnosis of bladder cancer and monitoring of patients for recurrence. We discovered a general cancer biomarker in blood and propose to develop a low cost rapid and non-invasive diagnostic test for BC diagnosis and monitoring in urine suitable for low resource settings.
STUDIES
SPECIFIC AIMS
Our objective is to develop a low cost non-invasive Rapid Diagnostic Test (RDT) for the detection of cancerous cells from urine based on an enzyme biomarker QSOX1 overexpressed in most cancers, including bladder, with which we will test urine samples from control and test populations. We will address this objective in three specific aims.
product development
IHC TEST
Immunohistochemistry (IHC) Diagnostic Test
AXIM is developing an IHC test using proprietary anti-QSOX1 polyclonal antibodies and novel monoclonal antibodies
results
PHASE TWO AND BEYOND
After our Phase I results, we hope to have developed a lateral flow assay that identifies the presence of QSOX1-L urine samples that we expect will correlate strongly with malignancy. After that, the test will be further optimized for FDA approval.