OVERVIEW

An Innovation in Dry Eye Disease Diagnosis
AXIM Eye has developed and commercialized two highly specialized Point-of-Care (POC) diagnostic tests designed specifically to assist eye-care physicians in detecting and quantifying biomarkers associated with aqueous deficient Dry Eye Disease and non-specific allergic conjunctivitis.

Rapid, Accurate & Non-Invasive
Both of AXIM’s currently offered tests provide rapid results (8 minutes), accuracy, and are non-invasive
Insurance & CMS Reimbursable
Both of AXIM’s tests are Medicare and insurance reimbursable, making them attractive to patients
Breakthrough DED Diagnosis
An estimated six million people have reported experiencing DED symptoms but have yet to be formally diagnosed or have received treatment
Current AXIM Eye Diagnostic Tests
Both of AXIM’s currently available tests are FDA cleared 510(k)s and insurance and CMS reimbursable. The tests are specific, accurate, non-invasive, and use tears as source analyte.

IgE
This test is designed for the measurement of Ocular Immunoglobulin E (IgE), a key biomarker primarily associated with non-specific, allergic conjunctivitis which often mimics DED.
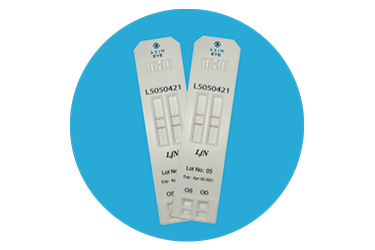
Lactoferrin
This is a rapid Point-of-Care (8 minute) lateral flow diagnostic assay paired with a reader that tests for exact levels of Lactoferrin through the collection of 0.5 microliter of tears.
Did you know?
The Axim Eye point-of-care Lactofferin and IgE diagnostic tests are CMS and private insurance reimbursable. Reimbursement rates can vary based on your practice location.
Lactoferrin CPT code – 83520
IgE. CPT code – 82785
Contact Axim Eye to obtain your own no charge personalized practice calculator.
