DIAGNOSTICS
AIM #1
Aim #1: Development and validation of polyclonal antibodies that detect only QSOX1-L isoform.
We will use two different approaches to generate polyclonal QSOX1-L specific antibodies. In the first approach, rabbits will be immunized with the entire recombinant 100aa C-terminal domain of QSOX1-L (Figure 4) purified from E. coli cells and conjugated to KLH. It is not known how C-terminal domain of QSOX1-L is processed in urine. Therefore, we decided to generate polyclonal antibodies to this entire domain, which will be used to detect all possible proteolytic products of QSOX1-L and serve as control and to deplete urine of QSOX1-L. In the second approach, we will use five 12-15aa chemically synthesized KLH-conjugated peptides (derived from the 100 aa C-terminal domain of QSOX1-L) as individual antigens (Figure 4). The antibodies against short 12-15aa peptides will be used in the assay development. Upon completion of immunization and evaluation of serum antibody titters
Protein expression and purification: The codon optimized version of cDNA encoding 100aa C-terminal domain with a cysteine residue at N-terminus will be synthesized de novo and cloned into pET23 E. coli expression vector (Sigma-Aldrich, USA) under the control of T7 RNA polymerase promoter with 6xHis-tag at C-terminus. Integrity of protein expression vector will be confirmed by DNA sequencing. The obtained vector will be transformed into E. coli BL21(DE3) cells. Transformed cells will be grown in liquid LB medium and recombinant protein expression will be induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG). Following SDS-PAGE confirmation and solubility test, protein will be purified by immobilized metal affinity chromatography (IMAC) either under native or denaturing conditions, followed by cation-exchange chromatography.
Peptide synthesis and conjugation: Five short peptides 12-15 (Figure 4) derived from 100aa C-terminal domain of QSOX1-L will be designed and made at a qualified peptide synthesis facility (GL Biochem, USA) at 95% purity and 10 mg scale. Each peptide will be conjugated to KLH for immunization and to bovine serum albumin (BSA) for serum titer evaluation and purified polyclonal antibodies tests, while unconjugated PEG3-linked peptides will be used for affinity column preparation. Recombinant 100aa C-terminal domain of QSOX1L and chemically synthesized peptides will be conjugated to KLH and BSA via heterobifunctional SMCC linker using Pierce kits PN 77605 and 77115, respectively, following manufacturer’s instructions.
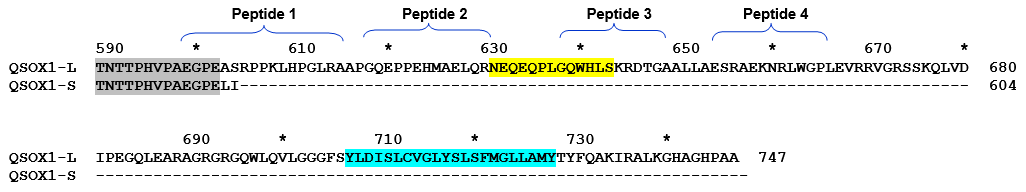
Figure 4. Primary structure of C-terminus of QSOX1-S and QSOX1-L and selection of peptides for polyclonal antibody development. Transmembrane domain of QSOX1-L is highlighted in blue. Peptide used for the development of polyclonal anti-NEQ antibody from Dr. Lake is highlighted in yellow.
Rabbits immunization: For each antigen, two 6-8 week old healthy female New Zealand White rabbits will be housed at a qualified animal facility (Abcore, Ramona, CA). Before primary immunization, 1-2ml of pre-immune serum samples will be collected as a control. For primary immunization, each rabbit will receive 100-200 µg dose of KLH-conjugated antigen emulsified with complete Freund’s adjuvant (CFA) via subcutaneous injection. Subsequent immunizations will be given every two weeks with antigen mixed in incomplete Freund’s adjuvant (IFA) via the same route. Approximately 1-2 ml of serum will be collected from immunized animals after each boost one week after each injection for serum antibody titer evaluation by indirect ELISA with corresponding antigens. Serum antibody titers are expected to be at least 1:100,000 after a total of 4 injections. Once the titers are reached, rabbits will continue to receive immunization boosts and approximately 20ml of serum form each animal will be collected 1 week after each injection for antibody purification.
Affinity purification and quality control: Affinity column for anybody purification will be prepared using Sulfo-link resin (Thermo Fisher PN 20401) reacted with cysteine-terminated peptides followed by serum purification per manufacturer’s instructions. Purified antibodies will be evaluated by indirect ELISA with corresponding peptide-BSA conjugate and recombinant QSOX1-L (received from D. Lake’ lab) in the presence of human urine depleted of QSOX1-L. Urine samples depleted of QSOX1-L will be prepared from pooled urine samples collected from healthy donors using 100aa antibody affinity column. Antibodies will be validated in Western blot assay.
Milestone: Six polyclonal antibodies specific to QSOX1-L that bind native QSOX1-L in urine are validated and ready to use in Aim #2.